Heat transfer in an air conditioning system:
The main characteristic of heat is that it moves from the hotter to the colder substance. This is easily recognisable when you put a cold kettle on a hot plate heat is being transferred from the hot substance (hot plate) to the cold substance (kettle) causing the kettle to heat up. The three main ways by which heat is transferred from substance to substance are known as 1) conduction 2) convection 3) and radiation.
1) Conduction is the mode by which heat is transferred in solid materials. The heat travels from particle to particle in the solid material causing each particle to heat up in turn. The rate at which each specific material can transfer heat depends on the thermal conductivity of each specific material. In general most heat exchanges are constructed from materials, which are good thermal conductors, and in the case of the condenser in an air conditioning system, this is no exception.
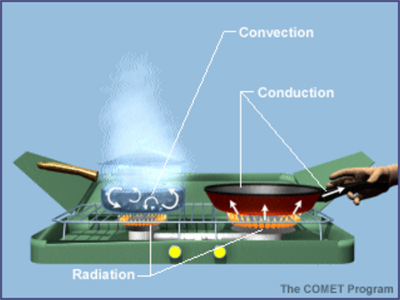
2) Convection is the heat transfer means by which the molecules or atoms of a liquid or gas move in a circular motion and carry the heat. If air which is hotter than the passenger compartment air is blown into the passenger compartment, it will move in the upwards direction forcing the cooler, more dense air downwards. When this type of air circulation forms, known as convection, it repeats itself it eventually distributes the warmer heated air evenly around the interior of the vehicle.
3) Radiation is the heat transfer means by which waves located in the infrared portion of the spectrum (electromagnetic) transfer heat with or without the use of a medium. Radiation is the only one of the three modes of heat transfer, which does not require a medium to transfer the heat along. You cannot see the waves, which transfer heat since they are in the invisible region of the electromagnetic spectrum. On a sunny day an automobile in effect acts as a miniature greenhouse that stores up the heat due to radiation.
These three main methods of heat transfer can now be used to describe the basic transfer of heat between and within an air conditioning system and the interior of a vehicle.
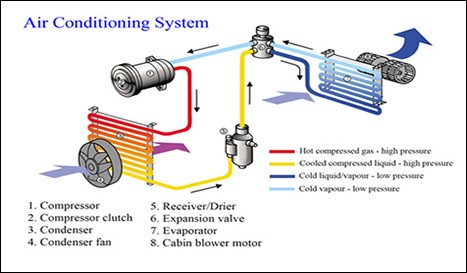
Air conditioning is quite similar to a refrigerant circuit in a fridge or freezer. It extracts heat from one place and releases it somewhere else. In the case of air conditioning it extracts heat from the passenger compartment in a vehicle and releases it into the atmosphere. It does this by the refrigerant in the air conditioning system absorbing the heat and then releasing it again. This is possible because the air conditioning system is able to manipulate three very important natural phenomena.
These include:
- Heat transfer which was also spoken about earlier
- The latent heat transfer of vaporization of a liquid
- And the effect pressure has on boiling or condensation of a liquid or gas
- Heat transfer occurs in two main places in an air conditioning system, in the evaporator in the passenger compartment and in the condenser
The heat transfer that occurs in the interior of a vehicle occurs between the refrigerant in gaseous form and the interior air. The cool refrigerant is taking heat away from the warmer passenger compartment, which in turn causes the passenger compartment to become cooler and the refrigerant to become hotter. The heat transfer externally in the condenser occurs between hotter liquid refrigerant and cooler ambient air (relative to the temp of the refrigerant liquid). Now since the air is cooler than the liquid refrigerant, heat is transferred from the liquid refrigerant to the air via the condenser causing the air to heat up and the refrigerant to loose heat and cool down. Heat since it is a form of energy is measured in kilojoules. In the case of water it takes 4.2 kJ to raise the temperature of 1 kg by 1ºC so conversely in order to reduce the temperature of 1 kg of water by 1ºC it will have to release 4.2 kJ of heat energy. The latent heat of a substance is the heat taken in or given out when changing state without changing temperature (the hidden heat). Therefore, it follows that the latent heat of vaporization is the heat taken in or given out when a substance is changing from liquid to gas without changing temperature. In the case of water it has a very large latent heat vaporization.
This is the same for many other liquids when evaporating from liquid to gas including the refrigerants in an air conditioning system. Using water as an example again, it takes 420 kJ of heat energy to raise the temp of 1 kg from 1ºC to 100ºC. At this point the water begins to boil (changing state from liquid to gas). Irrespective of how much more heat energy is applied at this point the temperature of the water will not increase until a complete state change has taken place from liquid to gas. Then and only then will the temperature of the water begin to increase again. It is this point that we are particularly interested in with respect to an air conditioning system. Refrigerants which are used in automobile air conditioning systems behave in a similar way to water with the exception that they boil at much lower temperatures than water (R134a boils at -27ºC) and they have got varying values for the specific heat capacity and also the latent heat vaporization depending on which refrigerant you are using. Water is not useable for one in an air conditioning system as a refrigerant since its boiling point is too high. Even at low pressure there is no real beneficial cooling effect that could be achieved when it evaporates. This is why special refrigerants with low boiling points are chosen.
The boiling point of each liquid is also dependent on pressure. The higher the pressure the higher the boiling point of the liquid. We now know that heat will be transferred from the hotter to the colder object/substance. In the air conditioning system the refrigerant is used as the medium through which heat is both taken in and given out. In order for the air conditioning system to be effective the boiling point of the refrigerant needs to be lower than that of the passenger compartment temperature and since the refrigerants generally boil between -25ºC and -30ºC they are able to absorb considerable amounts of heat from the passenger compartment. It is useless having a substance as refrigerant in an air conditioning system which has a boiling point at or near to the average ambient temperature since it will be unable to absorb a large amount of heat and the air conditioning system will neither be able to function effectively or efficiently.
